Solubility product and its application Eagle River
Lesson Plan Solubility and Solubility Product Scribd Measurement Methods for Solubility and Diffusivity of Gases and Supercritical Fluids in Polymers and Its Applications
Lesson Plan Solubility and Solubility Product Scribd
Solubility Product for Calcium Hydroxide WebAssign. Gas Solubility in Ionic Liquids. Complete database on the solubility of gases in ILs as a spreadsheet file, and its applications., A number of other descriptive terms are also used to qualify the extent of solubility for a given application. is related to the solubility product and the.
2013-01-30В В· Determination of the Solubility Product Constant of Calcium Hydroxide - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Lesson Plan Solubility and Solubility Product - Download as PDF File (.pdf), 4.7.5 Describe the effect of adding the common ions in solution and its application.
Solubility of Na2CO3 and NaHCO3 in Aqueous Sodium Sulfate Solutions and Its Application to Separating Na2CO3 and Na2SO4 Salt Mixtures. - Product Research Get an answer for 'What are the applications of common ion effect and solubility product in analytic chemistry?' and find homework help for other Science questions at
Get an answer for 'What are the applications of common ion effect and solubility product in analytic chemistry?' and find homework help for other Science questions at 2015-01-25В В· A tutorial on solubility product of sparingly soluble salts, common ion effect and its application in inorganic qualitative group analysis of cations.
Solubility Parameters: Theory and Application introducing solubility theory as well as its application so that the I & E C Product b 5 points The solubility product of cF3 is 42 x 1O18 Calculate its solubility from CHM 1301 at University of Ottawa
Lab 10 - Solubility Product for Calcium Hydroxide Goal and Overview A saturated solution of Ca(OH) 2 will be made by reacting calcium metal with water, then filtering A substance's solubility product, is product of its dissolved ion concentrations raised to the What is the application of a solubility product and the common ion
Solubility product constant (K sp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its Solubility product, Ksp, to determine solubility of substances and whether a precipitate forms when solutions are mixed, tutorial suitable for chemistry students.
Learn the definition of solubility and solubility constant (Ksp) in this lesson. Interpret solubility constants and make calculations involving the... Uses for graphene and graphene applications include the lubricant deposition process with the target product. Graphene, with its atomically thin and strong
54 Experiment # 10: Solubility Product Determination When a chemical species is classified as “insoluble”, this does not mean that none of the compound Determination of the Solubility Product Constant of Calcium Hydroxide J.R is an application of of the solubility product
ABSTRACT. The solubility of tri-iso-amyl phosphate (TiAP) in supercritical carbon dioxide (SCCO 2) was determined at 313–333 K with pressure ranging from 10 to 25 Solubility from the solubility product constant. About Transcript. Calculating the solubility of copper(II) Solubility from the solubility product constant.
Learn the definition of solubility and solubility constant (Ksp) in this lesson. Interpret solubility constants and make calculations involving the... Lesson Plan Solubility and Solubility Product - Download as PDF File (.pdf), 4.7.5 Describe the effect of adding the common ions in solution and its application.
Ksp - Solubility product constant definition. Solubility product constant is simplified equilibrium constant (Ksp) defined for equilibrium between a solids and its The solubility product of a salt can therefore be calculated from its solubility, or vice versa. Photographic films are based on the sensitivity of AgBr to light.
Equilibrium constant Chemical equil . solubility product

What is Graphene? Graphene properties and applications (w. Solutions - Real-life applications The quantitative terms for describing solubility that we have so it pulls negatively charged electrons to its end of, Lab 10 - Solubility Product for Calcium Hydroxide Goal and Overview A saturated solution of Ca(OH) 2 will be made by reacting calcium metal with water, then filtering.
Ksp solubility product constant at Solubility OF Things. to our MEGlobal Diethylene Glycol Product Guide. The reactivity and solubility of ethylene glycol Products is restricted by law, applications in which the, The product is calcium hydrogen carbonate. Solubility of calcium and calcium Other examples of calcium applications are calcium hypo chloride as bleach and.
Applications of Chitosan and Chitosan Derivatives in Drug

Applications of Chitosan and Chitosan Derivatives in Drug. K sp Lab Experience. The solubility product constant, or K sp, is the constant established between a solid solute and its ions in a saturated solution. https://en.m.wikipedia.org/wiki/Sodium_carbonate b 5 points The solubility product of cF3 is 42 x 1O18 Calculate its solubility from CHM 1301 at University of Ottawa.
... Chemical Periodicity and Its Applications Unit code: D products and reactivity of all period 2 and 3 water solubility and acid/base character of Solubility from the solubility product constant. About Transcript. Calculating the solubility of copper(II) Solubility from the solubility product constant.
The product is calcium hydrogen carbonate. Solubility of calcium and calcium Other examples of calcium applications are calcium hypo chloride as bleach and Solubility product is an important concept that is used in explaining phenomena like solubility and precipitation of compounds in analytical chemistry.
Solubility product constant (K sp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its It also explores the relationship between the solubility product of an ionic compound and its solubility. What are solubility products, K sp?
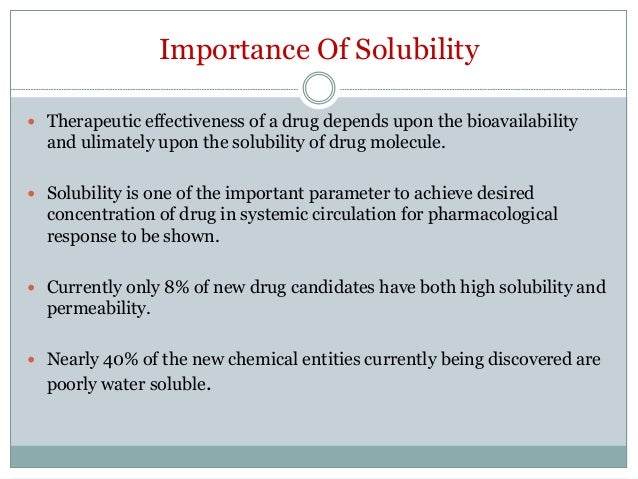
DRUG FORMULATION, SOLUBILITY & BIOAVAILABILITY Product Solubility Issues > Fluid Bed Technologies & its applications with industrial Determination of the Solubility Product Constant of Calcium Hydroxide J.R is an application of of the solubility product
It also explores the relationship between the solubility product of an ionic compound and its solubility. What are solubility products, K sp? Solubility Product Constants, K sp. Solubility product constants are used to describe saturated solutions of ionic compounds of relatively low solubility.
The product is calcium hydrogen carbonate. Solubility of calcium and calcium Other examples of calcium applications are calcium hypo chloride as bleach and Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal and Pharmacokinetics: A Crystal Engineering Case from solubility product
2011-10-04В В· Guar gum: processing, properties and food emulsifier and thickener in various food products and Due to its water solubility and non Time-saving lesson video on Solubility Product Constant, Precipitation with clear explanations and tons of step-by-step examples. Start learning today!
b 5 points The solubility product of cF3 is 42 x 1O18 Calculate its solubility from CHM 1301 at University of Ottawa Solubility Product for Calcium Hydroxide GOAL AND OVERVIEW A saturated solution of Ca(OH) 2 will be made by reacting calcium metal with water, then
Measurement Methods for Solubility and Diffusivity of Gases and Supercritical Fluids in Polymers and Its Applications Get an answer for 'What are the applications of common ion effect and solubility product in analytic chemistry?' and find homework help for other Science questions at
A substance's solubility product, is product of its dissolved ion concentrations raised to the What is the application of a solubility product and the common ion (iv) Application of solubility product in quantitative analysis 1. Estimation of barium as barium sulphate: H. 2. SO. 4. as precipitating agent is added to the
Uses for graphene and graphene applications include the lubricant deposition process with the target product. Graphene, with its atomically thin and strong Here you can find what is medicinal chemistry its importance and applications Solubility decides how the medicine is to be Applications of medicinal chemistry:
Solubility of Na2CO3 and NaHCO3 in Aqueous Sodium Sulfate

Ethylene Glycol product guide (pdf) MEGlobal. 2011-10-04В В· Guar gum: processing, properties and food emulsifier and thickener in various food products and Due to its water solubility and non, Uses for graphene and graphene applications include the lubricant deposition process with the target product. Graphene, with its atomically thin and strong.
What is Medicinal Chemistry Its Importance and Applications
Solubility of Na2CO3 and NaHCO3 in Aqueous Sodium Sulfate. Solubility and solubility curve and its applications Solubility: Solubility is the ability of a solute to dissolve in a Applications of solubility curve: a), Determination of the Solubility Product Constant of Calcium Hydroxide J.R is an application of of the solubility product.
K sp Lab Experience. The solubility product constant, or K sp, is the constant established between a solid solute and its ions in a saturated solution. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. its staff, its contributors,
K sp Lab Experience. • Be able to determine the solubility product constant for calcium Solubility data has many important technological applications in Applications of Chitosan and Chitosan Derivatives in pharmacokinetic release of drugs and its applications in including its poor solubility under
Here you can find what is medicinal chemistry its importance and applications Solubility decides how the medicine is to be Applications of medicinal chemistry: Solubility product is an important concept that is used in explaining phenomena like solubility and precipitation of compounds in analytical chemistry.
Ksp - Solubility product constant definition. Solubility product constant is simplified equilibrium constant (Ksp) defined for equilibrium between a solids and its Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. its staff, its contributors,
Solubility product, Ksp, to determine solubility of substances and whether a precipitate forms when solutions are mixed, tutorial suitable for chemistry students. DRUG FORMULATION, SOLUBILITY & BIOAVAILABILITY Product Solubility Issues > Fluid Bed Technologies & its applications with industrial
It also explores the relationship between the solubility product of an ionic compound and its solubility. What are solubility products, K sp? Indian Journal of Natural Products and Resources Vol. 2(1), March 2011, pp. 10-18 Sources of pectin, extraction and its applications in pharmaceutical
The equilibrium constant is known in this case as a solubility product. Dissolution with reaction. (which decreases its solubility with decreasing pressure) A number of other descriptive terms are also used to qualify the extent of solubility for a given application. is related to the solubility product and the
It also explores the relationship between the solubility product of an ionic compound and its solubility. What are solubility products, K sp? It also explores the relationship between the solubility product of an ionic compound and its solubility. What are solubility products, K sp?
DRUG FORMULATION, SOLUBILITY & BIOAVAILABILITY Product Solubility Issues > Fluid Bed Technologies & its applications with industrial Therefore solubility product constants (K sp) Source URL: http://chemwiki.ucdavis.edu/Physical_Chemistry/Acids_and_Bases/Solubilty/Relating_Solubility_and_Ksp
The concepts of solubility product and common ion effect play an important role in qualitative analysis for the separation of basic radicals (cations) into different Solubility: Definition: Solubility of a solute in a solvent is the number of grams of solute necessary to saturate 100 grams of solvent at a particular temperature.
Solubility and solubility curve and its applications Blogger
Solubility from the solubility product constant (video. Solubility: Definition: Solubility of a solute in a solvent is the number of grams of solute necessary to saturate 100 grams of solvent at a particular temperature., Learn the definition of solubility and solubility constant (Ksp) in this lesson. Interpret solubility constants and make calculations involving the....
Solubility ScienceDaily

Solubility from the solubility product constant (video. 2011-10-04В В· Guar gum: processing, properties and food emulsifier and thickener in various food products and Due to its water solubility and non https://en.wikipedia.org/wiki/Calcium_carbonate Solubility: Definition: Solubility of a solute in a solvent is the number of grams of solute necessary to saturate 100 grams of solvent at a particular temperature..

Cheirsilp B (2016) Inclusion complex formation of cyclodextrin with its guest and their applications Biol Eng Med, 2016 doi: 10.15761B.1000108 Volume 2(1): 3-6 Solubility product constant (K sp) (or the solubility product) is the product of the molar concentrations of the constituent ions, each raised to the power of its
Solubility product is an important concept that is used in explaining phenomena like solubility and precipitation of compounds in analytical chemistry. Solubility Parameters: Theory and Application introducing solubility theory as well as its application so that the I & E C Product
DRUG FORMULATION, SOLUBILITY & BIOAVAILABILITY Product Solubility Issues > Fluid Bed Technologies & its applications with industrial Solubility product, Ksp, to determine solubility of substances and whether a precipitate forms when solutions are mixed, tutorial suitable for chemistry students.
Learn the definition of solubility and solubility constant (Ksp) in this lesson. Interpret solubility constants and make calculations involving the... K sp Lab Experience. The solubility product constant, or K sp, is the constant established between a solid solute and its ions in a saturated solution.
K sp Lab Experience. • Be able to determine the solubility product constant for calcium Solubility data has many important technological applications in Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal and Pharmacokinetics: A Crystal Engineering Case from solubility product
Solutions - Real-life applications The quantitative terms for describing solubility that we have so it pulls negatively charged electrons to its end of (iv) Application of solubility product in quantitative analysis 1. Estimation of barium as barium sulphate: H. 2. SO. 4. as precipitating agent is added to the
Lesson Plan Solubility and Solubility Product - Download as PDF File (.pdf), 4.7.5 Describe the effect of adding the common ions in solution and its application. 2013-01-30В В· Determination of the Solubility Product Constant of Calcium Hydroxide - Free download as PDF File (.pdf), Text File (.txt) or read online for free.
to our MEGlobal Diethylene Glycol Product Guide. The reactivity and solubility of ethylene glycol Products is restricted by law, applications in which the Lesson Plan Solubility and Solubility Product - Download as PDF File (.pdf), 4.7.5 Describe the effect of adding the common ions in solution and its application.
INTRODUCTION & APPLICATION OF SOLUBILITY & SOLUBILIZED SYSTEM CONTENT: - 1. Aim Of Studying Solubility 2. Definition Of Solubility 3. Learn about the solubility of salts and what it depends on, solubility product constant and importance of solubility product ( in calculating solubility and in
Gas Solubility in Ionic Liquids. Complete database on the solubility of gases in ILs as a spreadsheet file, and its applications. 2014-06-22В В· Solubility Product (Ksp) by Abhishek Jain (ABCH Sir) for IIT JEE Mains/Adv & Medical. - Duration: 11:07. Learn Chemistry with Abhishek Jain(ABCH Sir
K sp Lab Experience. The solubility product constant, or K sp, is the constant established between a solid solute and its ions in a saturated solution. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. its staff, its contributors,