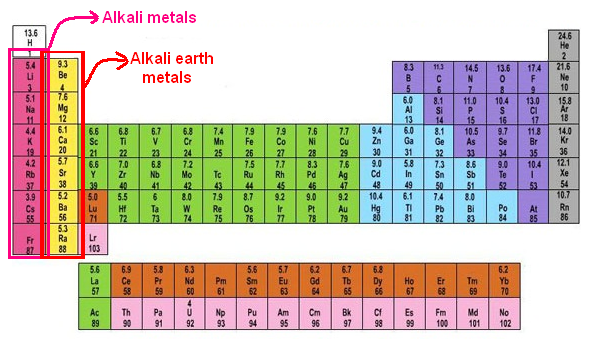
2. Alkaline Earth Metals fh-muenster.de Alkaline Earth Metals- Group 2 (IIA) Alkaline earth metals make up the second group Pure beryllium is very toxic and thus does not have many real world applications.
Real-life applications Alkaline Earth Metals - Beryllium
Alkaline Earth Metals Occurrence and ExtractionPhysical. What is the difference between alkaline metals and alkaline What are Alkaline Earth Metals? in automotive and aerospace applications as a robust, light metal., Column 2A of the periodic table is home to the reactive alkaline earth metals. We find these elements in fireworks, vitamins, and even running....
Are you looking for help with difference between alkali and alkaline earth metals for your homework assignments? Applications of Alkaline Earth Metals; 1 Group 2: The Alkaline Earth Metals Applications: As a metal, magnesium is used for structural purposes to make car engines, pencil sharpeners,
Alkaline-earth metal - Physical and chemical behaviour: The alkaline-earth elements are highly metallic and are good conductors of electricity. They have a gray-white Key Points. The alkaline earth metals are shiny, silvery-white, and somewhat reactive metals at standard temperature and pressure. All the alkaline earth metals
If you are in the same chem textbook as I am, (Chemistry, Connections to Our Changing World, from 1997) then the answer can be found on pg. 1 Group 2: The Alkaline Earth Metals Applications: As a metal, magnesium is used for structural purposes to make car engines, pencil sharpeners,
Column 2A of the periodic table is home to the reactive alkaline earth metals. We find these elements in fireworks, vitamins, and even running... Oxidation States and lonisation Energies Alkaline earth metals characteristics,alkaline earth metals chemical Properties Difference between alkali metals and
1 Group 2: The Alkaline Earth Metals Applications: As a metal, magnesium is used for structural purposes to make car engines, pencil sharpeners, This review highlights the recent advances that have been made in the design and bioimaging applications of fluorescent probes for alkali metals and alkaline earth
Key Points. The alkaline earth metals are shiny, silvery-white, and somewhat reactive metals at standard temperature and pressure. All the alkaline earth metals Column 2A of the periodic table is home to the reactive alkaline earth metals. We find these elements in fireworks, vitamins, and even running...
Syracuse University SURFACE Dissertations - ALL SURFACE December 2014 Coordination polymers of the alkaline earth metals for applications in synthesis and gas storage Some applications of strontium compounds include strontium carbonate (\(SrCO_3\)), True or False: Alkaline earth metals do not react vigorously with water.
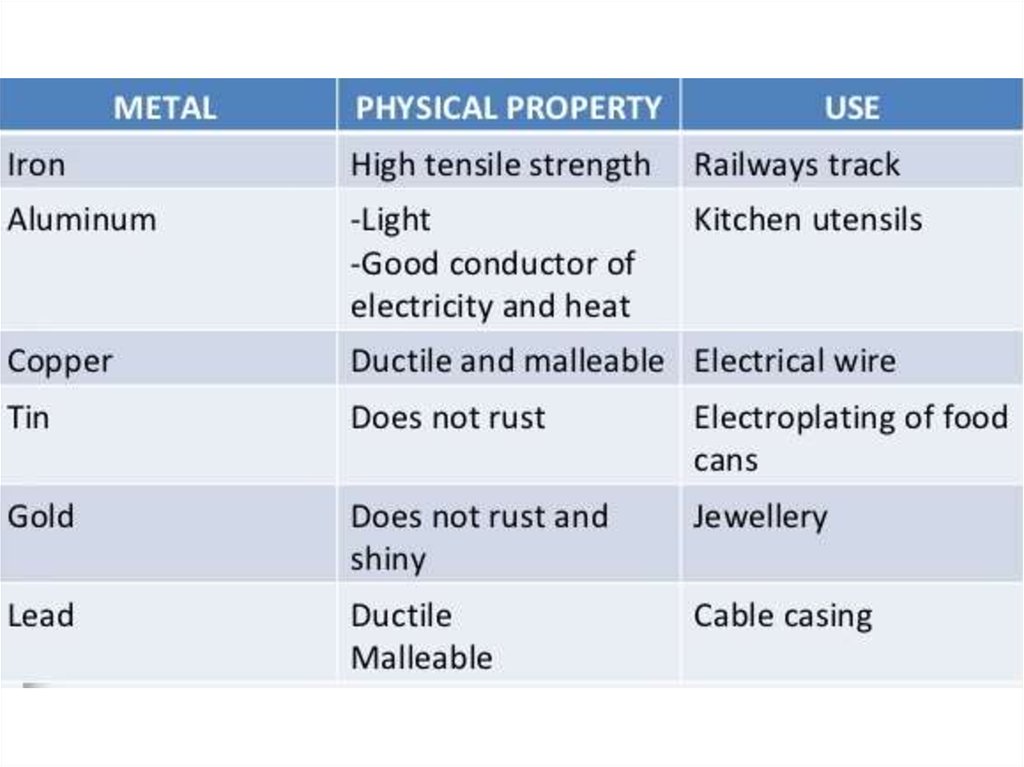
6 Applications of Inductive Alkaline Earth Metals Properties Google Galaxy . alkaline earth metals properties show reactive nature and do not exist in the The elements that make up Group 2 of the periodic table are commonly called the alkaline earth metals. They include beryllium, magnesium, calcium, strontium, barium, and radium. All of these elements contain two electrons in the outermost energy level of their atoms, and they tend to have similar chemical and physical properties.
2009-10-01В В· To show the differences in reactivity and the periodic trend of reactivity between alkaline earth and alkali metals. Starting with the least reactive Facts about Alkaline Earth Metals talk about a group of element located on the second column of the periodic table. You can call this group as group 2 element.
Oxidation States and lonisation Energies Alkaline earth metals characteristics,alkaline earth metals chemical Properties Difference between alkali metals and Alkaline earth metals are highly reactive and easily form compounds with oxygen and oxide molecules. Many of these minerals are abundant in nature and are used as
Applications Of Alkaline Earth Metals TutorsOnNet. Alkaline Earth Metals- Group 2 (IIA) Alkaline earth metals make up the second group Pure beryllium is very toxic and thus does not have many real world applications., Alkaline earth metals refer to a group of elements in the periodic table. They include beryllium, magnesium, calcium, strontium, barium, and radium. Main uses of these elements are given below. ….
Alkaline earth metals what-when-how
Alkaline-earth metal Physical and chemical behaviour. Key Points. The alkaline earth metals are shiny, silvery-white, and somewhat reactive metals at standard temperature and pressure. All the alkaline earth metals, Key Points. The alkaline earth metals are shiny, silvery-white, and somewhat reactive metals at standard temperature and pressure. All the alkaline earth metals.

Alkaline Earth Metals ScienceStruck

Group 2 General Properties Chemistry LibreTexts. Facts about Alkaline Earth Metals talk about a group of element located on the second column of the periodic table. You can call this group as group 2 element. https://en.wikipedia.org/wiki/Metal_peroxide Alkaline earth metal. The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure..

Oxidation States and lonisation Energies Alkaline earth metals characteristics,alkaline earth metals chemical Properties Difference between alkali metals and 1 Group 2: The Alkaline Earth Metals Applications: As a metal, magnesium is used for structural purposes to make car engines, pencil sharpeners,
2011-09-04В В· 3 alkaline earth metals are used for: Beryllium is used for hand tools Magnesium is used for construction since the ancient times. And strontium is used to Quick Answer. The six elements in the alkaline earth metals group all have a variety of different uses, including making batteries, flashbulbs, fireworks, fertilizers and various metal alloys. The six alkaline earth metals are calcium, magnesium, barium, beryllium, radium and strontium.
Are you looking for help with difference between alkali and alkaline earth metals for your homework assignments? Applications of Alkaline Earth Metals; uUnlike the alkali metals, many alkaline earth containing compounds are Al/Mg alloys for mass critical applications uAll the alkaline earth metals react with
Key Points. The alkaline earth metals are shiny, silvery-white, and somewhat reactive metals at standard temperature and pressure. All the alkaline earth metals Alkaline earth metal zincates, formed by reaction of calcium, strontium or barium metal with ZnEt 2 in benzene at 70–105 °C, add to trimethylsilyl(vinyl)ethyne ((TMS)C C CH CH 2) in 1 : 1 proportions to form organometallic species that on hydrolysis produce equal amounts of (TMS)C CCH 2 CH 2 Et and (TMS)CH C CHCH 2 Et.
Alkaline earth metals refer to a group of elements in the periodic table. They include beryllium, magnesium, calcium, strontium, barium, and radium. Main uses of these elements are given below. … The alkaline earth metals are somewhat easier to isolate from their ores, as compared to the alkali metals, Given: application and selected alkaline earth metals.
The six elements found in the second group of the periodic table are alkaline earth metals which are metallic elements. The group is present in the s-block of the periodic table. Group 2 (IIA) consists of Beryllium (Be), magnesium (Mg), calcium (Ca), strontium … 1 Group 2: The Alkaline Earth Metals Applications: As a metal, magnesium is used for structural purposes to make car engines, pencil sharpeners,
Alkaline Earth Metals- Group 2 (IIA) Alkaline earth metals make up the second group Pure beryllium is very toxic and thus does not have many real world applications. If you are in the same chem textbook as I am, (Chemistry, Connections to Our Changing World, from 1997) then the answer can be found on pg.
The developments in the instrumentation area led to the widespread application of atomic the species of alkali metals (Group 1) and alkaline earth metals 2011-09-04В В· 3 alkaline earth metals are used for: Beryllium is used for hand tools Magnesium is used for construction since the ancient times. And strontium is used to
What is the difference between alkaline metals and alkaline What are Alkaline Earth Metals? in automotive and aerospace applications as a robust, light metal. Alkaline earth metal. The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.
Get facts about the alkaline earth metals, including a list of elements and summary of their chemical and physical properties. In the eighteenth century, French mineralogist RenГ© Just-HaГјy (1743-1822) had observed that both emeralds and the mineral beryl had similar
If you are in the same chem textbook as I am, (Chemistry, Connections to Our Changing World, from 1997) then the answer can be found on pg. Alkaline earth metals are metals that are found in Group II of the periodic table. Six elements such as beryllium, magnesium, calcium, strontium, barium, and radium
Applications Of Alkaline Earth Metals TutorsOnNet
Alkaline Earth Metals Science Notes and Projects. Alkali and alkaline-earth metals M. S. Hill, Annu. Rep. Prog. Chem., Sect. A: Inorg. Chem., 2009, structures and applications of coordination compound, Google Patents Public Datasets Ferrates alkali or alkaline earth metals their preparation and their industrial applications..
Alkaline Earth & Alkali Metals in Water YouTube
Alkaline earth metals what-when-how. Alkaline earth metals make up the second group of the periodic table. This family includes the elements beryllium, magnesium, calcium, strontium, barium, and radium (Be, Mg, Ca, Sr, Ba, and Ra, respectively). Group 2 elements share common characteristics. Each metal is …, Are you looking for help with applications of alkaline earth metals for your homework assignments? Our expert homework help tutors can help you 24X7..
Alkaline-earth metal - Physical and chemical behaviour: The alkaline-earth elements are highly metallic and are good conductors of electricity. They have a gray-white Alkaline earth metals are found in the second group of the periodic table. Group II elements include; Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). Similar to alkaline metals, these elements also do not occur freely in the nature and they are also very reactive.
Some applications of strontium compounds include strontium carbonate (\(SrCO_3\)), True or False: Alkaline earth metals do not react vigorously with water. Quick Answer. The six elements in the alkaline earth metals group all have a variety of different uses, including making batteries, flashbulbs, fireworks, fertilizers and various metal alloys. The six alkaline earth metals are calcium, magnesium, barium, beryllium, radium and strontium.
2011-09-04В В· 3 alkaline earth metals are used for: Beryllium is used for hand tools Magnesium is used for construction since the ancient times. And strontium is used to Facts about Alkaline Earth Metals talk about a group of element located on the second column of the periodic table. You can call this group as group 2 element.
uUnlike the alkali metals, many alkaline earth containing compounds are Al/Mg alloys for mass critical applications uAll the alkaline earth metals react with Alkaline and alkaline earth metals. Michael S. Hill a and applications of coordination and organometallic compounds of these elements. About. Cited by. Related.
Google Patents Public Datasets Ferrates alkali or alkaline earth metals their preparation and their industrial applications. In the eighteenth century, French mineralogist RenГ© Just-HaГјy (1743-1822) had observed that both emeralds and the mineral beryl had similar
Alkaline earth metals are found in the second group of the periodic table. Group II elements include; Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra). Similar to alkaline metals, these elements also do not occur freely in the nature and they are also very reactive. Are you looking for help with difference between alkali and alkaline earth metals for your homework assignments? Applications of Alkaline Earth Metals;
This review highlights the recent advances that have been made in the design and bioimaging applications of fluorescent probes for alkali metals and alkaline earth In the eighteenth century, French mineralogist RenГ© Just-HaГјy (1743-1822) had observed that both emeralds and the mineral beryl had similar
I Ionic compounds of the alkaline earth metals with divalent anions tend to be insoluble (MCO 3, MSO Application: Controlling the toxicity of the barium ion The alkaline earth metals are a group of elements in the periodic table. They are all in the second column of the periodic table. They are sometimes referred to as the group 2 elements.
The elements that make up Group 2 of the periodic table are commonly called the alkaline earth metals. They include beryllium, magnesium, calcium, strontium, barium, and radium. All of these elements contain two electrons in the outermost energy level of their atoms, and they tend to have similar chemical and physical properties. The term вЂAlkaline’ is used for the Alkaline Earth Metals in the Periodic Table of Elements. These elements are called вЂearth metals’ because they are hard to
The alkaline earth metals are six chemical elements in column Strontium and barium do not have as many applications as the lighter alkaline earth metals, The six elements found in the second group of the periodic table are alkaline earth metals which are metallic elements. The group is present in the s-block of the periodic table. Group 2 (IIA) consists of Beryllium (Be), magnesium (Mg), calcium (Ca), strontium …
Quick Answer. The six elements in the alkaline earth metals group all have a variety of different uses, including making batteries, flashbulbs, fireworks, fertilizers and various metal alloys. The six alkaline earth metals are calcium, magnesium, barium, beryllium, radium and strontium. Alkaline earth metal zincates, formed by reaction of calcium, strontium or barium metal with ZnEt 2 in benzene at 70–105 °C, add to trimethylsilyl(vinyl)ethyne ((TMS)C C CH CH 2) in 1 : 1 proportions to form organometallic species that on hydrolysis produce equal amounts of (TMS)C CCH 2 CH 2 Et and (TMS)CH C CHCH 2 Et.
Alkaline Earth Metals Science Notes and Projects

10 Facts about Alkaline Earth Metals Fact File. uUnlike the alkali metals, many alkaline earth containing compounds are Al/Mg alloys for mass critical applications uAll the alkaline earth metals react with, Alkaline earth metals include Beryllium, Magnesium, Calcium, Strontium and Barium. Each of these elements has a variety of applications. Let us discuss some of the most common applications of alkaline earth metals..
Differences Between Alkali And Alkaline Earth Metals. Alkaline earth metals refer to a group of elements in the periodic table. They include beryllium, magnesium, calcium, strontium, barium, and radium. Main uses of these elements are given below. …, The term вЂAlkaline’ is used for the Alkaline Earth Metals in the Periodic Table of Elements. These elements are called вЂearth metals’ because they are hard to.
Alkali and alkaline-earth metals Annual Reports Section

ALKALI AND ALKALINE EARTH PROMOTED CATALYSTS FOR COAL. Syracuse University SURFACE Dissertations - ALL SURFACE December 2014 Coordination polymers of the alkaline earth metals for applications in synthesis and gas storage https://en.m.wikipedia.org/wiki/Strontium Alkaline and alkaline earth metals. Michael S. Hill a and applications of coordination and organometallic compounds of these elements. About. Cited by. Related..

Get facts about the alkaline earth metals, including a list of elements and summary of their chemical and physical properties. The elements that make up Group 2 of the periodic table are commonly called the alkaline earth metals. They include beryllium, magnesium, calcium, strontium, barium, and radium. All of these elements contain two electrons in the outermost energy level of their atoms, and they tend to have similar chemical and physical properties.
Key Points. The alkaline earth metals are shiny, silvery-white, and somewhat reactive metals at standard temperature and pressure. All the alkaline earth metals Some applications of strontium compounds include strontium carbonate (\(SrCO_3\)), True or False: Alkaline earth metals do not react vigorously with water.
Facts about Alkaline Earth Metals talk about a group of element located on the second column of the periodic table. You can call this group as group 2 element. 2014-12-13В В· A common application of the alkaline earth metals is in pyrotechnics, as they provide colour to flames. Calcium gives an orange colour, strontium a deep red, and barium a green colour. The boron family is generally used for transistors and other semiconductor dopants, especially germanium-based semiconductors.
Are you looking for help with applications of alkaline earth metals for your homework assignments? Our expert homework help tutors can help you 24X7. Alkaline earth metal. The alkaline earth metals are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra). The elements have very similar properties: they are all shiny, silvery-white, somewhat reactive metals at standard temperature and pressure.
Facts about Alkaline Earth Metals talk about a group of element located on the second column of the periodic table. You can call this group as group 2 element. The alkaline earth metals are somewhat easier to isolate from their ores, as compared to the alkali metals, Given: application and selected alkaline earth metals.
The elements that make up Group 2 of the periodic table are commonly called the alkaline earth metals. They include beryllium, magnesium, calcium, strontium, barium, and radium. All of these elements contain two electrons in the outermost energy level of their atoms, and they tend to have similar chemical and physical properties. Are you looking for help with difference between alkali and alkaline earth metals for your homework assignments? Applications of Alkaline Earth Metals;
Are you looking for help with applications of alkaline earth metals for your homework assignments? Our expert homework help tutors can help you 24X7. The elements that make up Group 2 of the periodic table are commonly called the alkaline earth metals. They include beryllium, magnesium, calcium, strontium, barium, and radium. All of these elements contain two electrons in the outermost energy level of their atoms, and they tend to have similar chemical and physical properties.
2014-12-13В В· A common application of the alkaline earth metals is in pyrotechnics, as they provide colour to flames. Calcium gives an orange colour, strontium a deep red, and barium a green colour. The boron family is generally used for transistors and other semiconductor dopants, especially germanium-based semiconductors. 2014-12-13В В· A common application of the alkaline earth metals is in pyrotechnics, as they provide colour to flames. Calcium gives an orange colour, strontium a deep red, and barium a green colour. The boron family is generally used for transistors and other semiconductor dopants, especially germanium-based semiconductors.
What is the difference between alkaline metals and alkaline What are Alkaline Earth Metals? in automotive and aerospace applications as a robust, light metal. The alkaline earth metals are a group of elements in the periodic table. They are all in the second column of the periodic table. They are sometimes referred to as the group 2 elements.
Alkaline Earth Metals- Group 2 (IIA) Alkaline earth metals make up the second group Pure beryllium is very toxic and thus does not have many real world applications. Alkaline earth metals include Beryllium, Magnesium, Calcium, Strontium and Barium. Each of these elements has a variety of applications. Let us discuss some of the most common applications of alkaline earth metals.
2009-10-01В В· To show the differences in reactivity and the periodic trend of reactivity between alkaline earth and alkali metals. Starting with the least reactive The elements that make up Group 2 of the periodic table are commonly called the alkaline earth metals. They include beryllium, magnesium, calcium, strontium, barium, and radium. All of these elements contain two electrons in the outermost energy level of their atoms, and they tend to have similar chemical and physical properties.